L1: Charge Carriers and Doping
In our previous classes we had various devices that we could play with including and not limited to: resistors, capacitors, and inductors. We will call these "Basic Electronic Components" and if we were only given these devices to create things we would find it very difficult to create anything useful. This is the main reason that in most of the circuit theory classes you may have taken there were not many examples of useful circuits.
In this series we will lead up to talking about a bit more complicated electronic devices such as diodes, transistors(BJT & MOS), and op amps. With these devices available we can design much more sophisticated and useful circuits. However, because these devices are based on Semiconductors, we need to first understand semiconductors from a physics and chemistry standpoint.
In this series we will lead up to talking about a bit more complicated electronic devices such as diodes, transistors(BJT & MOS), and op amps. With these devices available we can design much more sophisticated and useful circuits. However, because these devices are based on Semiconductors, we need to first understand semiconductors from a physics and chemistry standpoint.
Semiconductor Physics General Concepts
All atoms are composed of a nucleus, and surrounding electrons. The nucleus is composed of just protons and neutrons. A common question to ask is
Let's take a look at 3 elements: Sodium, Neon, and Silicon. Sodium has a single electron in it's valence shell making it extremely reactive. Neon however, has its valence shell full of 8 electrons making it very neutral/Inert (ie. a Noble Gas). If we look at Silicon we can notice that it has 4 electrons in its valence shell so it has the possibility of wanting to react with other elements.
Let's take a look at 3 elements: Sodium, Neon, and Silicon. Sodium has a single electron in it's valence shell making it extremely reactive. Neon however, has its valence shell full of 8 electrons making it very neutral/Inert (ie. a Noble Gas). If we look at Silicon we can notice that it has 4 electrons in its valence shell so it has the possibility of wanting to react with other elements.
Silicon Crystals
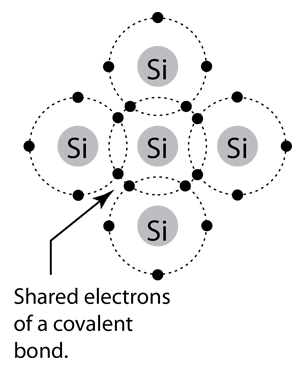
A Silicon Crystal also known as "Lattice" is an array of silicon atoms very neatly arranged next to each other as seen in Fig 1. You can see that each silicon atom decides to share its 4 electrons with each of the silicon atoms next to it to complete its valence shell.
At this point we are probably wondering if a silicon crystal is able to conduct electricity? So let's say that we hook up two the sides of the crystal two voltage leads and we will try to measure the current drawn form the crystal. In order for a current to move we would need some electrons to be free from a covalent bond to freely move from the higher voltage potential to the lower voltage potential. However, based on this model there should be 0 current flow since none of the electrons in the silicon are free. And if you were to perform this test in real life you would also find this to be true at absolute zero degrees C (at other temperatures electrons may come off of their bonds and become free allowing for some amount of current to pass through). So you can see at certain temperatures Silicon can conduct, it may not conduct as well as some other metals, but it acts as an insulator near absolute zero. At room temperature the conductivity of silicon is about 10^-10 times less than a metallic conductor, but also 10^14 times larger than that of an insulator. Pure Silicon (aka Intrinsic Silicon) has 5 x 10^22 atoms per cubic centimeter, it also has one free electron for conduction for every 5 x 10^12 silicon atoms. Thus, intrinsic silicon has about 10^10 electrons/cm^3 that are available for conduction.
Doping: Increasing the Conductivity of Semiconductors
From what we have mentioned thus far it is apparent that pure silicon is not a very good conductor. To increase the conductivity of silicon we need to increase the electrons available for conduction. One way to increase the conductivity of silicon is to heat the material in order to provide greater number of charge carriers inside the semiconductor. For example, for every 25 degrees Celsius in temperature, the conductivity rises by a factor of about ten. Shining light on semiconductors also increases the number of free electrons (this is how photo voltaic cells work). However, the most effective way to attain a desired conductivity for a semiconductor is to carefully introduce impurities into the material which is a process known as Doping.
By introducing impurities of antimony or phosphorous (n-type impurities), we can increase the number of negative carriers, or electrons into the semiconductor. In fact, when as little as 1 silicon atom in 10 million silicon atoms is replaced with an n-type impurity atom, the conductivity of silicon at room temperature increases by a factor of about 10^5. The level of doping is roughly proportional to the number of impurity atoms per unit volume.
A semiconductor is unusual in the way that electrons are not the only charge carriers. In intrinsic silicon there are positively charge carriers known as holes that contribute the conductivity of the material as well. In intrinsic silicon the number of holes is equal to the number of electrons available. Thus, at room temperature intrinsic silicon has about 10^10 holes/cm^3. Holes can be added to Silicon using p-type impurities such as Boron. Specifically, if we added 10^16 boron atoms/cm^3 to silicon's 5 x 10^22 atom/cm^3, the concentration holes increases to 10^16 holes per cubic centimeter or an increase of 10^6 times.
N-Type Doping vs. P-Type Doping
Quantitatively, doping with n-type impurities versus p-type impurities have the same affect on the conductivity. For example, the addition 1 n-type impurity atom will have almost the same affect as 1 p-type impurity. However, if equal parts n-type and p-type impurities are added into intrinsic silicon, there will be no noticeable change as the n-type impurity and p-type impurity will negate each others affects as the free electrons from the n-type will recombine with the holes of the p-type impurity.